<はじめに>
暇すぎてPubmed巡回(特定の研究デザインの論文を片っ端から読む。このブログはニッチさがウリ?なのでネットワークメタ解析なんかをよく探しています)をしていたら、バロキサビル(ゾフルーザ)のネットワークメタ解析の論文を発見したので、「CAPSTONE1の内容でネットワークメタしてる暇あるんやったら、はよCAPSTONE2(ハイリスク患者対象の試験)論文化してよ!」と思いながら読んでみた。
注意:今回記事がやたら長いので読む際にはご注意ください!
(暇な人以外ここでブラウザをそっ閉じすることを推奨します)
<宣伝>
CAPSTONE1の過去記事はこちら
zuratomo4.hatenablog.com
<お題論文>
A network meta-analysis of the efficacy and safety of baloxavir marboxil versus neuraminidase inhibitors for the treatment of influenza in otherwis... - PubMed - NCBI
PMID: 30810054
チェックシート改訂しましたので以下のリンクからどうぞ
Dropbox - ネットワークメタ解析チェックシートVer9.pdf
<読んでみる>
①リサーチクエスチョンの特定
アブストラクトから
P:合併症のないインフルエンザ患者
I/C:バロキサビル/他の抗インフルエンザ薬
O:有効性、安全性
本文メソッドから
P:合併症のないインフルエンザ患者
I/C:バロキサビル(80㎏未満40㎎、80㎏以上80㎎)、オセルタミビル1回75㎎1日2回5日、ザナミビル1回10㎎1日2回5日、ラニナミビル40㎎、ペラミビル300㎎、プラセボ
O:有効性→症状緩和までの時間、解熱までの時間(平熱への回復時間)、ウイルス排出停止までの時間、ウイルス力価低下(24時間、48時間)、インフルエンザ発症前の状態への回復時間(通常活動ができるまでの時間)、肺炎
安全性→有害事象、薬剤関連有害事象、嘔吐、下痢
※リレンザ開発前にあったザナミビル鼻噴霧スプレーは解析に入っていない模様。
②システマティックレビューの評価
・データベース:MEDLINE、EMBASE、CENTRAL、Clinicaltrials.gov、EMA、FDA、PMDA、学会抄録
・検索語:薬剤成分名、薬剤開発コード、influenza、RCT
・検索日:2016.11.14(CAPSTONE1以外の論文がやけに古いのはこのため)
・元論文:RCTのみ収集しているが、個々のRCTの質の評価をした形跡はない
・評価者:二者(独立しているかはきちんと書いていないが独立でやっていそう)→意見対立時は第三者介入
・出版:言語制限の有無は不明。参考文献はおっているが、専門家への連絡はなし(そもそもラストオーサーのHirotsu N.先生が専門家のような…)。ファンネルプロットは実行できるほどの研究数がない。
・異質性:事前登録はない。PやOはよくある設定なので統合できないということはなさそう(特に第二相臨床試験が対象になりそう)
⇒元論文バイアスと出版バイアスはかかっているとみてよさそう
※おまけ:Hirotsu N.先生は以下のような記事でよく見る先生。
廣津伸夫:最近話題のキーワード:日経メディカル Online
インフルエンザ、家庭でどう広がる? データにあらわれた意外な結果 | ハフポスト
1回飲むだけのインフル新薬「ゾフルーザ」ってどんな薬? 医師に聞いた | ハフポスト
③ネットワークメタ解析の評価
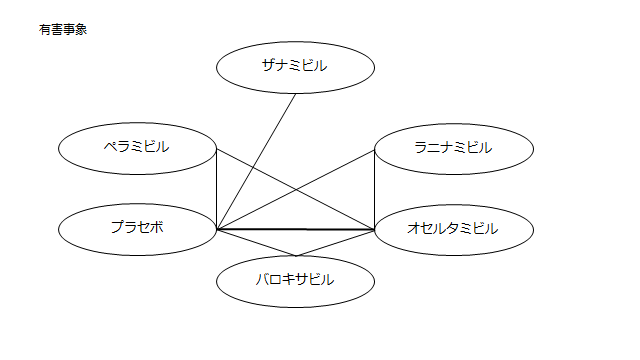
・ネットワーク図:まとめたものが一つ示されている。(一つ一つ確認しないと個々のアウトカムの評価ができないが、複数テーブルに該当研究が示されており見づらい)
・閉じた環:少ない
・研究数、サンプルサイズ:プラセボvsザナミビル、プラセボvsオセルタミビル以外は1~2研究しかない
※IGLOO試験(ラニナミビルの欧州第二相試験https://clinicaltrials.gov/ct2/show/record/NCT01793883)が入っていないが、これは検索日が2016.11だから(IGLOOの結果がNCTに載ったのが2017.9.1)日本語で解説が見たい方は以下のリンクよりどうぞ。
http://ph-minimal.hatenablog.com/entry/2018/11/15/023743
・直接比較、間接比較:分けて記載していないので評価できない
資金源:公開されている。塩野義製薬が資金を出してCreativ-Ceutical社に依頼している
※Creativ-Ceutical社https://www.creativ-ceutical.com/:HTAなどの医療技術系の資料作成などをしている会社のよう。日本にもあり代表のかたの論文やセミナー情報がある。
https://www.jstage.jst.go.jp/article/jjpe/23/1/23_49/_pdf/-char/en
https://tech-seminar.jp/lecturer/%E5%A4%A7%E8%A5%BF-%E4%BD%B3%E6%81%B5
COI:公開されているが塩野義関連のもののみ。
④結果(対バロキサビルで表示されている。㎎数は1日用量。数値はMDで表記。太字は有意差あり)
・症状緩和までの時間
プラセボ:29.36(15.34~45.82)
ザナミビル20㎎:19.96(3.23~39.07)
ラニナミビル40㎎:6.41(-15.48~39.07)
オセルタミビル150㎎:6.33(-6.89~19.54)
ペラミビル300㎎:7.60(-8.49~24.78)
・解熱までの時間
プラセボ:19.12(6.44~33.25)
ザナミビル20㎎:4.65(-20.06~41.56)
ラニナミビル40㎎:0.41(-18.82~24.20)
オセルタミビル150㎎:-1.14(-14.16~10.33)
ペラミビル300㎎:-1.69(-17.50~14.48)
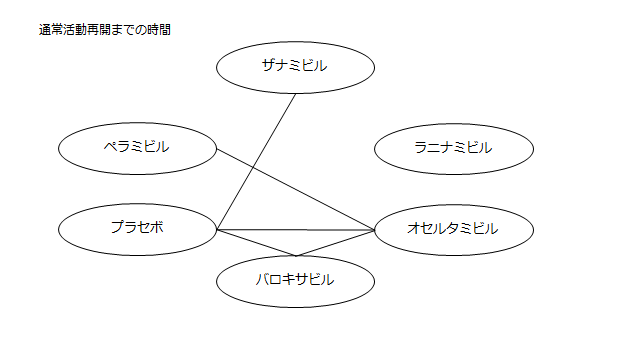
・インフルエンザ発症前の状態への回復時間
プラセボ:33.25(-24.07~90.96)
ザナミビル20㎎:18.77(-61.58~107.20)
ラニナミビル40㎎:NR
オセルタミビル150㎎:-6.88(-70.17~54.81)
ペラミビル300㎎:0.84(-77.97~77.66)
・ウイルス排出停止までの時間
プラセボ:84.04(-50.65~131.90)
ザナミビル20㎎:47.00(28.18~73.86)
ラニナミビル40㎎:NR
オセルタミビル150㎎:56.03(33.74~87.86)
ペラミビル300㎎:NR
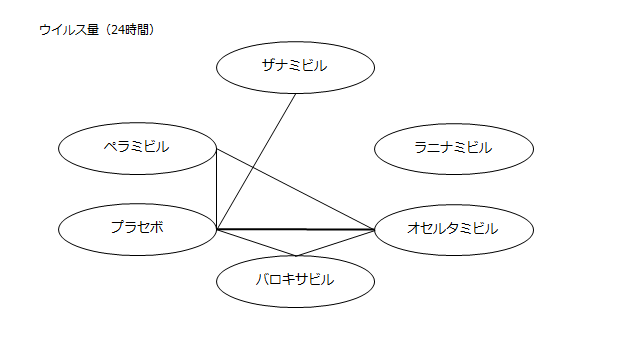
・ウイルス力価低下(24時間)
プラセボ:2.98(2.10~3.90)
ザナミビル20㎎:2.49(1.12~3.85)
ラニナミビル40㎎:NR
オセルタミビル150㎎:2.30(1.32~3.30)
ペラミビル300㎎:2.31(1.19~3.49)
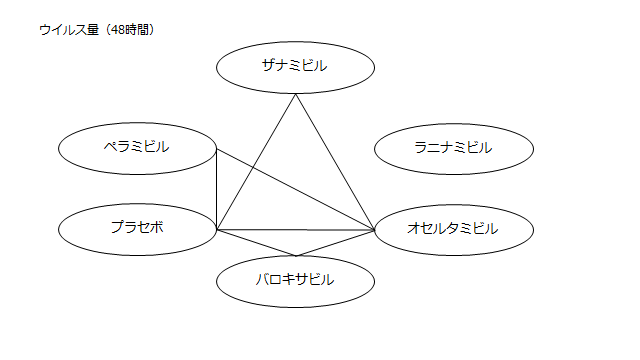
・ウイルス力価低下(48時間)
プラセボ:1.64(0.82~2.49)
ザナミビル20㎎:1.17(0.11~2.28)
ラニナミビル40㎎:NR
オセルタミビル150㎎:0.74(-0.15~1.67)
ペラミビル300㎎:1.06(0.02~2.14)
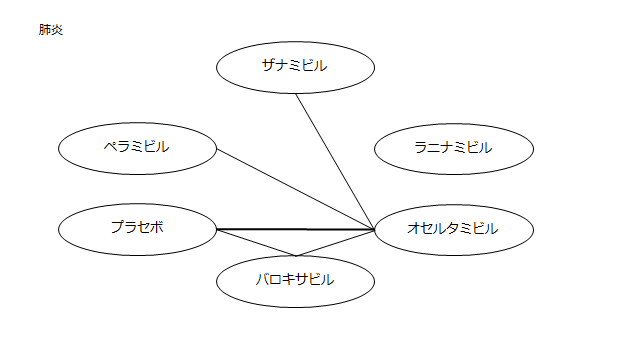
・肺炎(ここからOR)
プラセボ:0.92(0.07~90.96)
ザナミビル20㎎:0.13(0.0002~15.37)
ラニナミビル40㎎:NR
オセルタミビル150㎎:0.44(0.03~4.86)
ペラミビル300㎎:0.71(0.03~17.36)
・有害事象
プラセボ:1.28(0.96~1.69)
ザナミビル20㎎:1.01(0.72~1.42)
ラニナミビル40㎎:1.02(0.65~1.57)
オセルタミビル150㎎:1.12(0.85~1.47)
ペラミビル300㎎:1.02(0.70~1.50)
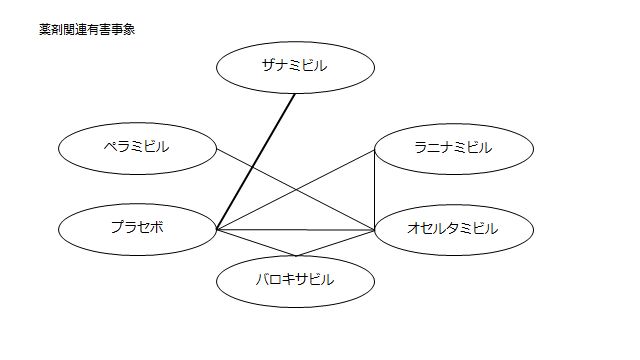
・薬剤関連有害事象
プラセボ:1.15(0.67~1.95)
ザナミビル20㎎:1.03(0.55~1.92)
ラニナミビル40㎎:2.02(1.05~3.99)
オセルタミビル150㎎:1.92(1.18~3.20)
ペラミビル300㎎:1.25(0.67~2.36)
・嘔吐
プラセボ:0.91(0.38~2.19)
ザナミビル20㎎:NR
ラニナミビル40㎎:0.64(0.24~1.77)
オセルタミビル150㎎:0.70(0.30~1.68)
ペラミビル300㎎:0.51(0.17~1.57)
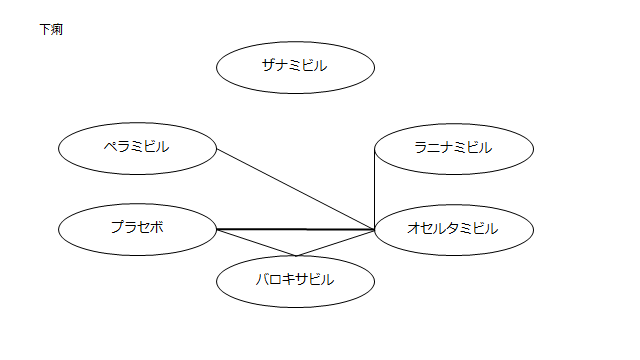
・下痢
プラセボ:0.65(0.12~5.41)
ザナミビル20㎎:NR
ラニナミビル40㎎:0.21(0.01~3.35)
オセルタミビル150㎎:2.42(0.48~19.26)
ペラミビル300㎎:0.26(0.02~3.10)
<まとめ>
多くの治験で主要評価項目となっている症状緩和までの時間で、バロキサビルはプラセボやザナミビルに勝ったというが、試験選定に問題がありそう。
また、研究数も少なくこれからの結果次第で容易に覆りそう。
本記事作成中に以下のような報道があり、「もうすでに結果変わりそう(´;ω;`)」と涙目になりました。
https://medical.nikkeibp.co.jp/leaf/mem/pub/hotnews/int/201903/560176.html
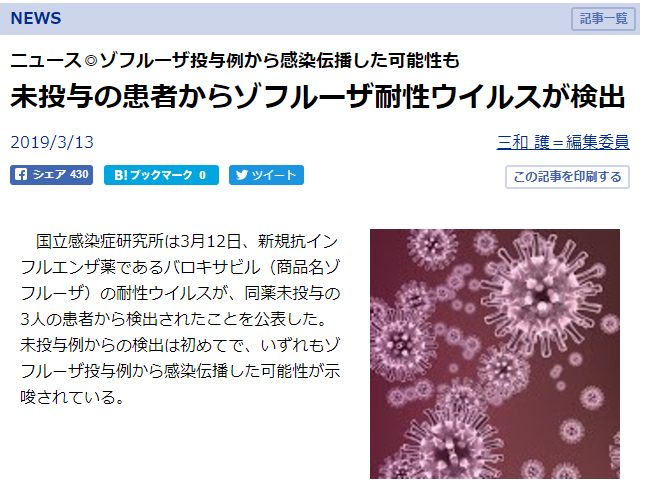
<論文では不十分だった情報を埋められるだけ埋めてみた>
実際に集まった研究のうち読めるものを読んでrisk of biasを評価してみた。
(評価はかなり甘目。ただし全く触れていない場合は第三相試験だから大丈夫だろうと思ってもunclearとした)
L=low risk of bias U=unclear of bias H=high risk of bias
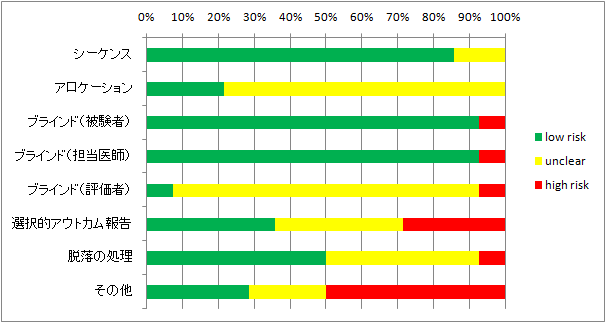
第二相、第三相が主なので、集まった研究の質は良いほうだと思う。
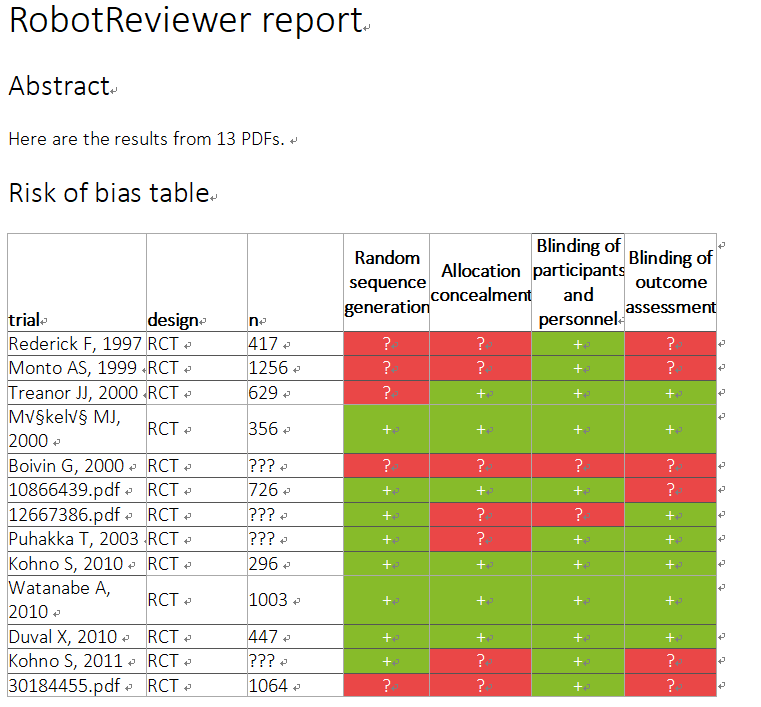
自分の評価では心もとないのでRobotReviewerにも評価してもらった。(なんか色が変)(trial部分が数字になっているのはPMID)
Characteristics of studies
Rederick F, 1997
|
Population
|
- Patients Previously healthy persons who were at least 18 years old (at least 13 years in North America) with an acute influenza-like illness of 48 hours' duration during documented influenzavirus circulation in the community were enrolled.
- We assessed the therapeutic activity of zanamivir in adults with acute influenza.
- Conclusions In adults with influenza A or B virus infections, direct administration of a selective neuraminidase inhibitor, zanamivir, to the respiratory tract is safe and reduces symptoms if begun early.
|
|
Intervention
|
- A total of 417 adults with influenza-like illness of 48 hours' duration were randomly assigned to one of three treatments: 6.4 mg of zanamivir by intranasal spray plus 10 mg by inhalation, 10 mg of zanamivir by inhalation plus placebo spray, or placebo by both routes.
- Patients were randomly assigned to receive one of three treatments: 10 mg of zanamivir by inhalation by mouth plus 6.4 mg by intranasal spray, 10 mg of zanamivir by inhalation plus placebo nasal sprays, or placebo by both routes.
- Results Of 262 patients with confirmed influenzavirus infection (63 percent of all patients), the median length of time to the alleviation of all major symptoms was one day shorter (four days vs. five days) in the 88 patients given inhaled and intranasal zanamivir (P 0.02) and the 85 patients given inhaled zanamivir alone (P 0.05) than in the 89 patients given placebo.
|
|
Outcomes
|
- The primary clinical end point was the length of time to the alleviation of all major symptoms of influenza, as defined by the absence of feverishness and the presence of no other major symptoms (headache, myalgia, cough, and sore throat), or only mild ones, for at least 24 hours.
- Among the infected patients who were febrile at enrollment and among those who began treatment within 30 hours after the onset of symptoms , the median time to the alleviation of major symptoms was four days in both zanamivir groups and seven days in the placebo group (P 0.01).
- Severity was rated on a four-point scale in which a score of 0 indicated no symptoms, a score of 1 mild symptoms, a score of 2 moderate symptoms, and a score of 3 severe symptoms .
|
|
Bias
|
Judgement
|
Support for judgement
|
|
Random sequence generation
|
high/unclear
|
- Patients were randomly assigned to receive one of three treatments: 10 mg of zanamivir by inhalation by mouth plus 6.4 mg by intranasal spray, 10 mg of zanamivir by inhalation plus placebo nasal sprays, or placebo by both routes.
- A total of 417 adults with influenza-like illness of 48 hours' duration were randomly assigned to one of three treatments: 6.4 mg of zanamivir by intranasal spray plus 10 mg by inhalation, 10 mg of zanamivir by inhalation plus placebo spray, or placebo by both routes.
- Methods We conducted separate randomized, double-blind studies in 38 centers in North America and 32 centers in Europe during the influenza season of 1994–1995.
|
|
Allocation concealment
|
high/unclear
|
- Methods We conducted separate randomized, double-blind studies in 38 centers in North America and 32 centers in Europe during the influenza season of 1994–1995.
- Patients were randomly assigned to receive one of three treatments: 10 mg of zanamivir by inhalation by mouth plus 6.4 mg by intranasal spray, 10 mg of zanamivir by inhalation plus placebo nasal sprays, or placebo by both routes.
- 14 Pairwise comparisons of intranasal and inhaled zanamivir with placebo and of inhaled zanamivir with placebo were performed with an extended Mantel–Haenszel test, with integer scores stratified according to the protocol.
|
|
Blinding of participants and personnel
|
low
|
- Both were randomized, double-blind, and placebo-controlled in design and tested the same regimen of drug treatment.
- Methods We conducted separate randomized, double-blind studies in 38 centers in North America and 32 centers in Europe during the influenza season of 1994–1995.
- The secondary end points were analyzed in the same manner as the primary end point, except for the viral shedding AUC, which was analyzed with analysis of covariance to allow for effects due to base line (day 1 value) and treatment.
|
|
Blinding of outcome assessment
|
high/unclear
|
- 14 Pairwise comparisons of intranasal and inhaled zanamivir with placebo and of inhaled zanamivir with placebo were performed with an extended Mantel–Haenszel test, with integer scores stratified according to the protocol.
- The patients recorded their symptoms (nasal stuffiness or runny nose, sore throat, cough, muscle aches, tiredness or fatigue, headache , loss of appetite, and feverishness) on a diary card each morning and evening.
- Other demographic characteristics of enrolled patients and their severity of illness were generally similar (Table 1 ), although an excess of smokers was present in the group assigned to intranasal and inhaled zanamivir.
|
Monto AS, 1999
|
Population
|
- Subjects ages 65 years or with the following chronic illnesses were included and regarded as being at high risk for developing complications or having more severe or prolonged illness: cardiovascular conditions (excluding hypertension), respiratory conditions , and endocrine or metabolic conditions.
- Otherwise healthy persons ages 13 years who presented with symptoms of influenza of 48 h duration were enrolled in the study during a single influenza season (November 1995 through March 1996 ).
- Pregnant or breast-feeding women and women at risk of becoming pregnant during the study were also excluded.
|
|
Intervention
|
- Subjects were randomized in the ratio 2:2:1:1 to receive one of the following treatments for 5 days: zanamivir, 10 mg 2/day by oral inhalation plus 6.4 mg 2/day by nasal spray; zanamivir, 10 mg 4/day by oral inhalation plus 6.4 mg 4/day by nasal spray; placebo by both routes 2/day; or placebo by both routes 4/day.
- The study was a double-blind randomized placebo-controlled multicenter parallel-group study that compared the efficacy and safety of zanamivir administered 2 or 4 a day for treatment of influenza A and B infections.
- The efficacy and safety of zanamivir, administered 2 or 4 daily over 5 days, was evaluated in the treatment of influenza infections.
|
|
Outcomes
|
- The primary clinical end point was the time to alleviation of clinically significant symptoms, defined as the absence of feverishness, a temperature !37.8C, and a score of 0 (none) or 1 (mild) for other major symptoms (i.e., headache, myalgia , sore throat, and cough), which had to be maintained 24 h. Time to alleviation was measured in half-days from the start of treatment (day 1), with the morning of the first day of treatment corresponding to 0 days.
- Secondary end points included mean symptom score, sleep turbance, time to return to normal activities, and use of acetaminophen and cough mixture to relieve symptoms.
- The primary end point, " al-leviation of major symptoms, " was created to evaluate differences in clinical impact.
|
|
Bias
|
Judgement
|
Support for judgement
|
|
Random sequence generation
|
high/unclear
|
- Subjects were randomized in the ratio 2:2:1:1 to receive one of the following treatments for 5 days: zanamivir, 10 mg 2/day by oral inhalation plus 6.4 mg 2/day by nasal spray; zanamivir, 10 mg 4/day by oral inhalation plus 6.4 mg 4/day by nasal spray; placebo by both routes 2/day; or placebo by both routes 4/day.
- In total, 1258 persons were enrolled in the study, and 1256 persons were randomized to one of the treatment groups as follows: zanamivir, 2/day, 419; zanamivir, 4/day, 415; placebo, 422 (figure 1).
- The study was doubleblind to active treatment versus placebo but not for dosing schedule .
|
|
Allocation concealment
|
high/unclear
|
- Subjects were randomized in the ratio 2:2:1:1 to receive one of the following treatments for 5 days: zanamivir, 10 mg 2/day by oral inhalation plus 6.4 mg 2/day by nasal spray; zanamivir, 10 mg 4/day by oral inhalation plus 6.4 mg 4/day by nasal spray; placebo by both routes 2/day; or placebo by both routes 4/day.
- Medication was selfadministered , and subjects were instructed to take the inhaled medication before the intranasal medication.
- The study was doubleblind to active treatment versus placebo but not for dosing schedule .
|
|
Blinding of participants and personnel
|
low
|
- The study was doubleblind to active treatment versus placebo but not for dosing schedule .
- Subjects were randomized in the ratio 2:2:1:1 to receive one of the following treatments for 5 days: zanamivir, 10 mg 2/day by oral inhalation plus 6.4 mg 2/day by nasal spray; zanamivir, 10 mg 4/day by oral inhalation plus 6.4 mg 4/day by nasal spray; placebo by both routes 2/day; or placebo by both routes 4/day.
- The study was a double-blind randomized placebo-controlled multicenter parallel-group study that compared the efficacy and safety of zanamivir administered 2 or 4 a day for treatment of influenza A and B infections.
|
|
Blinding of outcome assessment
|
high/unclear
|
- The study was a double-blind randomized placebo-controlled multicenter parallel-group study that compared the efficacy and safety of zanamivir administered 2 or 4 a day for treatment of influenza A and B infections.
- Subjects were randomized in the ratio 2:2:1:1 to receive one of the following treatments for 5 days: zanamivir, 10 mg 2/day by oral inhalation plus 6.4 mg 2/day by nasal spray; zanamivir, 10 mg 4/day by oral inhalation plus 6.4 mg 4/day by nasal spray; placebo by both routes 2/day; or placebo by both routes 4/day.
- The study was doubleblind to active treatment versus placebo but not for dosing schedule .
|
Treanor JJ, 2000
|
Population
|
- Participants Previously healthy adults aged 18 to 65 years who presented within 36 hours of onset of influenza symptoms and who had documented oral temperature of 38°C or higher at enrollment plus 1 or more respiratory symptom (cough, sore throat, or nasal symptoms ) and 1 or more constitutional symptom (headache, malaise, myalgia , sweats and/or chills, or fatigue) were enrolled.
- Individuals were excluded from the study if they had received influenza vaccination in the 12 months prior to the beginning of the study; had active, clinically significant chronic illness or human immunodeficiency virus disease; were receiving systemic steroids or other immunosuppressants; or had a history of alcohol or drug abuse.
|
|
Intervention
|
- Drug Administration Participants were randomly assigned to 1 of 3 treatment groups: oseltamivir, 75 mg or 150 mg orally twice daily, or matching placebo for 5 days.
|
|
Outcomes
|
- Participants Previously healthy adults aged 18 to 65 years who presented within 36 hours of onset of influenza symptoms and who had documented oral temperature of 38°C or higher at enrollment plus 1 or more respiratory symptom (cough, sore throat, or nasal symptoms ) and 1 or more constitutional symptom (headache, malaise, myalgia , sweats and/or chills, or fatigue) were enrolled.
- Clinical Monitoring Participants recorded the severity of 7 influenza symptoms (cough, nasal obstruction , sore throat, fatigue, headache , myalgia, and feverishness) using a 4-point scale (0, absent; 3, severe) twice daily for 21 days.
- For the primary efficacy analysis, laboratory-documented influenza infection was defined as isolation of influenza virus from nasal secretions and/or a 4-fold or greater HAI antibody re- sponse.
|
|
Bias
|
Judgement
|
Support for judgement
|
|
Random sequence generation
|
high/unclear
|
- Drug Administration Participants were randomly assigned to 1 of 3 treatment groups: oseltamivir, 75 mg or 150 mg orally twice daily, or matching placebo for 5 days.
- Randomization occurred at the time of study entry by telephone contact with an automated service that had sole access to the code key and was stratified by study site and smoking behavior.
- Participants and staff remained blinded to allocation status throughout the study.
|
|
Allocation concealment
|
low
|
- Randomization occurred at the time of study entry by telephone contact with an automated service that had sole access to the code key and was stratified by study site and smoking behavior.
- Participants and staff remained blinded to allocation status throughout the study.
- Drug Administration Participants were randomly assigned to 1 of 3 treatment groups: oseltamivir, 75 mg or 150 mg orally twice daily, or matching placebo for 5 days.
|
|
Blinding of participants and personnel
|
low
|
- Participants and staff remained blinded to allocation status throughout the study.
- Drug Administration Participants were randomly assigned to 1 of 3 treatment groups: oseltamivir, 75 mg or 150 mg orally twice daily, or matching placebo for 5 days.
- All laboratory tests were performed by individuals blinded to study assignment.
|
|
Blinding of outcome assessment
|
low
|
- The scales were demonstrated to be easily comprehended by English-speaking volunteers and correlate well with other questions about activity and with influenza symptom scores (Influenza Questionnaire Pilot Study Report, August 1997, Hoffmann-La Roche, data on file).
- Randomization occurred at the time of study entry by telephone contact with an automated service that had sole access to the code key and was stratified by study site and smoking behavior.
- Following this, they were asked to record their assessment of health status at baseline and over a 24-hour period once daily in the evening.
|
Mäkelä MJ, 2000
|
Population
|
- Introduction Even in patients with uncomplicated self-limiting influenza, the symptoms of the disease can be severe and incapacitating, often confining patients to bed while the fever is present, with associated absenteeism from school or work.
|
|
Intervention
|
- Patients were randomized (1:1) in a double-blind fashion to receive zanamivir 10 mg inhaled orally twice daily via a Diskhaler TM or matching placebo for 5 days.
- Patients aged ‚â• 12 years were recruited within 2 days of onset of typical influenza symptoms and received orally inhaled zanamivir 10 mg via a Diskhaler TM twice daily for 5 days or matching placebo.
|
|
Outcomes
|
- Other endpoints included symptom severity, use of relief medications, time to return to normal activities, complications and investigator's assessment of symptoms.
- Zanamivir significantly reduced the time to alleviation of symptoms versus placebo (median 5 days versus 7.5 days, P < 0.001), a 33% reduction in duration of illness.
- The primary endpoint was the time until alleviation of clinically significant symptoms of influenza, defined as no fever (temperature < 37.8°C and feverishness recorded as 'none') and headache, muscle or joint aches and pains, cough and sore throat recorded as 'none' or 'mild', maintained for 24 h (three consecutive diary card readings).
|
|
Bias
|
Judgement
|
Support for judgement
|
|
Random sequence generation
|
low
|
- The randomization code was generated using an internal Glaxo Wellcome program.
- Because of the unpredictable nature of influenza outbreaks, some centres would inevitably recruit small numbers of patients; therefore, each patient was randomized independently, with no stratification for centre.
- Patients were randomized (1:1) in a double-blind fashion to receive zanamivir 10 mg inhaled orally twice daily via a Diskhaler TM or matching placebo for 5 days.
|
|
Allocation concealment
|
low
|
- Because of the unpredictable nature of influenza outbreaks, some centres would inevitably recruit small numbers of patients; therefore, each patient was randomized independently, with no stratification for centre.
- The randomization code was generated using an internal Glaxo Wellcome program.
- The study medication was packed in individually numbered treatment packs.
|
|
Blinding of participants and personnel
|
low
|
- Patients were randomized (1:1) in a double-blind fashion to receive zanamivir 10 mg inhaled orally twice daily via a Diskhaler TM or matching placebo for 5 days.
- This was a randomized, double-blind, placebo-controlled trial in primary care and hospital clinics in 11 European countries.
- The study medication was packed in individually numbered treatment packs.
|
|
Blinding of outcome assessment
|
low
|
- Patients were randomized (1:1) in a double-blind fashion to receive zanamivir 10 mg inhaled orally twice daily via a Diskhaler TM or matching placebo for 5 days.
- 24 Patients who had missing diary card data and no positive evidence of alleviation were assigned as treatment failures and were included in the analysis as being alleviated after the end of the study.
- The assumptions did not appear justified for these data.
|
Boivin G, 2000
|
Population
|
- The antiviral and clinical effects of inhaled zanamivir (10 mg twice daily for 5 days, started within the first or second day of a flulike illness) were evaluated in a randomized, placebo-controlled trial during the 1997–1998 influenza season in Canada.
|
|
Intervention
|
- The antiviral and clinical effects of inhaled zanamivir (10 mg twice daily for 5 days, started within the first or second day of a flulike illness) were evaluated in a randomized, placebo-controlled trial during the 1997–1998 influenza season in Canada.
- This was a double-blind, randomized, placebo-controlled multicenter study conducted during the 1997–1998 winter to investigate the efficacy and safety of inhaled zanamivir (Glaxo Wellcome, Mississauga, Canada) at a dosage of 10 mg twice daily for 5 days in the treatment of symptomatic influenza virus infections [5].
- After only 12 h of treatment (1 dose), median virus titers decreased by 1.0 log 10 TCID 50 /mL in the zanamivir group (), compared with a 0.42-log 10 increase in the n = 17 placebo group (;).
|
|
Outcomes
|
- The primary end point of the clinical trial was the length of time to alleviation of all clinically important symptoms, as defined by no fever and other flu symptoms recorded as absent or mild for at least 24 h. Laboratory procedures.
- This was associated with a 4.5-day (47.4%) reduction in the n = 10 P = .08 median time to alleviation of all significant flu symptoms in the zanamivir recipients (P = after adjusting for the initial virus titer and the time between onset of symptoms and .03 treatment).
- Patients' symptoms and fever were self-assessed on a diary card by use of a 4-point scale 4 times daily during treatment and then twice daily for another 9 days.
|
|
Bias
|
Judgement
|
Support for judgement
|
|
Random sequence generation
|
high/unclear
|
- The antiviral and clinical effects of inhaled zanamivir (10 mg twice daily for 5 days, started within the first or second day of a flulike illness) were evaluated in a randomized, placebo-controlled trial during the 1997–1998 influenza season in Canada.
- The NA and hemagglutinin (HA)–1 genes of selected H3N2 virus isolates were reverse-transcribed and PCR-amplified (C-Therm Polymerase One- Step RT-PCR System; Boehringer, Mannheim, Germany) with NA primers 14/1420 [8] or HA1 primers 7/1184 [9].
- Serial swabs were then obtained by a research nurse every 12 h, prior to each dose of zanamivir.
|
|
Allocation concealment
|
high/unclear
|
- The antiviral and clinical effects of inhaled zanamivir (10 mg twice daily for 5 days, started within the first or second day of a flulike illness) were evaluated in a randomized, placebo-controlled trial during the 1997–1998 influenza season in Canada.
- Swabs were immediately placed in 2 mL of a virus transportation medium (Cellmatics; Difco, Detroit) and kept at 4C for a maximum of 36 h before cell inoculation in a central laboratory in Québec.
- Eight 10-fold dilutions were inoculated in quadruplicate onto MDCK cells in 24-well plates to determine the TCID 50 .
|
|
Blinding of participants and personnel
|
high/unclear
|
- Again, all paired isolates from those patients had identical HA1 sequences with the exception of patient 15.
- This was a double-blind, randomized, placebo-controlled multicenter study conducted during the 1997–1998 winter to investigate the efficacy and safety of inhaled zanamivir (Glaxo Wellcome, Mississauga, Canada) at a dosage of 10 mg twice daily for 5 days in the treatment of symptomatic influenza virus infections [5].
- The median time to alleviation of all flu symptoms for influenza virus–positive subjects was 9.5 days in the placebo group and 5.0 days in the zanamivir group (47.4% difference; ).
|
|
Blinding of outcome assessment
|
high/unclear
|
- The antiviral and clinical effects of inhaled zanamivir (10 mg twice daily for 5 days, started within the first or second day of a flulike illness) were evaluated in a randomized, placebo-controlled trial during the 1997–1998 influenza season in Canada.
- This was a double-blind, randomized, placebo-controlled multicenter study conducted during the 1997–1998 winter to investigate the efficacy and safety of inhaled zanamivir (Glaxo Wellcome, Mississauga, Canada) at a dosage of 10 mg twice daily for 5 days in the treatment of symptomatic influenza virus infections [5].
- Thirty-five patients were enrolled in the trial; of these, 27 (77%) had an influenza virus infection laboratory-confirmed by both culture and PCR (100% concordance on day 1).
|
10866439.pdf
|
Population
|
- We excluded patients who had been vaccinated against influenza in the previous 12 months, had active clinically important chronic illness or known HIV-1 infection, were receiving steroids or other immunosuppressants, and who had a history of drug or alcohol abuse.
- Eligible patients were aged 18–65 years and presented within 36 h of onset of influenza-like illness with fever of at least 38°C, with at least one respiratory symptom (cough, sore throat, or nasal symptom) and at least one constitutional symptom Articles For personal use only.
|
|
Intervention
|
- Patients were randomly assigned oseltamivir 75 mg, oseltamivir 150 mg, or matching placebo twice daily for 5 days.
- Patients were assigned oral oseltamivir 75 mg (n=243), oseltamivir 150 mg (n=245), or placebo (n=238) twice daily for 5 days.
- Discussion The administration of oral oseltamivir 75 mg or 150 mg twice daily was associated with significant clinical and antiviral effects in healthy adults with naturally occurring influenza and was generally well tolerated.
|
|
Outcomes
|
- Symptom relief was taken to occur at the start of the first 24 h period in which all influenza symptoms were scored as mild or none and remained so for at least 24 h. Other endpoints included: time to resolution of influenzal illness for all randomised patients; severity of illness, defined as the area under the curve for total symptom scores, for the whole illness duration; health, activity, and sleep quality, defined as the area under curve for scale scores, for the whole the treatment period; the frequency of and need for antibiotic treatment for common complications of influenza (otitis media, bronchitis, sinusitis, and pneumonia); and virus shedding.
- Assessments Patients recorded their oral temperature and the presence and severity of influenza symptoms of cough, nasal obstruction, sore throat, fatigue, headache, myalgia, and feverishness on a fourpoint scale (0 absent, 1 mild, 2 moderate, and 3 severe) twice daily for up to 21 days.
- The primary efficacy endpoint was the length of time to resolution of influenzal illness (defined as the period from start of study-drug to relief of symptoms) in the intention-to-treat population of infected patients.
|
|
Bias
|
Judgement
|
Support for judgement
|
|
Random sequence generation
|
low
|
- Randomisation was computer generated by a central randomisation facility, which had sole access to the code.
- Each centre provided its own medication in individually numbered packs, according to the instructions of the randomisation centre.
- Patients were randomly assigned oseltamivir 75 mg, oseltamivir 150 mg, or matching placebo twice daily for 5 days.
|
|
Allocation concealment
|
low
|
- Randomisation was computer generated by a central randomisation facility, which had sole access to the code.
- Each centre provided its own medication in individually numbered packs, according to the instructions of the randomisation centre.
- Patients were randomly assigned oseltamivir 75 mg, oseltamivir 150 mg, or matching placebo twice daily for 5 days.
|
|
Blinding of participants and personnel
|
low
|
- Randomisation was computer generated by a central randomisation facility, which had sole access to the code.
- Patients were randomly assigned oseltamivir 75 mg, oseltamivir 150 mg, or matching placebo twice daily for 5 days.
- Median virus titre areas under curves were lower by 30–40% during the first 4 days of treatment in the oseltamivir groups than in the placebo group (75 mg group, 78·2 log 10 TCID 50 h/mL, p=0·03; 150 mg group, 94·4 log 10 TCID 50 h/mL, p=0·003; placebo group, 130·8 log 10 TCID 50 h/mL).
|
|
Blinding of outcome assessment
|
high/unclear
|
- These assays were done by standard methods with antigens known to be circulating during the 1997–98 influenza season (influenza A/Shenzen/95 [H1N1], A/Wuhan/95 [H3N2], A/Sydney/97 [H3N2], and B/Harbin/95).
- We did not design this study to compare the activity of the two dose groups; the effects did not differ between doses for magnitude of clinical and antiviral effects.
- We did a double-blind randomised placebocontrolled study to investigate the efficacy and safety of oseltamivir in the treatment of naturally acquired influenza in human beings.
|
12667386.pdf
|
Population
|
- .
|
|
Intervention
|
- .
|
|
Outcomes
|
- .
|
|
Bias
|
Judgement
|
Support for judgement
|
|
Random sequence generation
|
low
|
- .
- J3% 6/8/0'()*% '00%*)'()5$ 475457()5$ 5-(3% 51%0( ;
|
|
Allocation concealment
|
high/unclear
|
- .
- J3% 6/8/0'()*% '00%*)'()5$ 475457()5$ 5-(3% 51%0( ;
|
|
Blinding of participants and personnel
|
high/unclear
|
- .
- J3% 6/8/0'()*% '00%*)'()5$ 475457()5$ 5-(3% 51%0( ;
|
|
Blinding of outcome assessment
|
low
|
- .
- J3% 6/8/0'()*% '00%*)'()5$ 475457()5$ 5-(3% 51%0( ;
|
Puhakka T, 2003
|
Population
|
- Patients who met any of the following criteria were excluded: hypersensitive to any component of the study medication or paracetamol, immunocompromised, currently using antibiotics for respiratory tract infection, had used influenza or any other antiviral therapy within 7 d of the study.
- PATIENTS AND METHODS Patients
- Women of childbearing potential were required to use acceptable methods of contraception and to have a negative urine test for pregnancy.
|
|
Intervention
|
- Eligible patients were randomly assigned by means of a computer-generated randomization schedule in blocks of 6 to receive 2 inhalations of either zanamivir (Relenza‚Ñ¢, GlaxoSmithKline; 5 mg per inhalation) or matched placebo (lactose powder) twice daily (morning and evening) for 5 d: a total daily dose of 20 mg.
- Conscripts were recruited within 2 d of onset of typical influenza symptoms and received inhaled zanamivir 10 mg via a Diskhaler‚Ñ¢ twice daily for 5 d or matching placebo.
- A randomized, double-blind, placebo-controlled, parallel-group trial performed at 5 residential units of the Finnish Defence Forces was conducted to assess the antiviral activity, efficacy and safety of inhaled zanamivir for the treatment of naturally acquired influenza.
|
|
Outcomes
|
- The symptoms recorded (using a 4-point severity scale: 0, for no symptoms; 1, mild; 2, moderate; and 3, severe) included headache, sore throat, feverishness, cough, muscle/joint aches and pains, nasal symptoms, weakness, loss of appetite and overall influenza-related symptom assessment.
- Patients were required to have an influenza-like illness (of less than 48 h duration ), defined as the presence of fever (temperature ] 37.8°C) and at least 2 of the following symptoms: headache, muscle/joint aches and pain, sore throat and cough.
- Assessment of illness Patients recorded the severity of their influenza symptoms, oral temperature using a digital thermometer (Becton-Dickinson AccuBeep standard digital thermometer) and use of relief medication 4 times daily for the first 3 d then twice daily until day 28 on diary cards.
|
|
Bias
|
Judgement
|
Support for judgement
|
|
Random sequence generation
|
low
|
- Eligible patients were randomly assigned by means of a computer-generated randomization schedule in blocks of 6 to receive 2 inhalations of either zanamivir (Relenza‚Ñ¢, GlaxoSmithKline; 5 mg per inhalation) or matched placebo (lactose powder) twice daily (morning and evening) for 5 d: a total daily dose of 20 mg.
- A randomized, double-blind, placebo-controlled, parallel-group trial performed at 5 residential units of the Finnish Defence Forces was conducted to assess the antiviral activity, efficacy and safety of inhaled zanamivir for the treatment of naturally acquired influenza.
- This was a randomized, double-blind, placebo-controlled, parallelgroup trial performed at 6 garrisons of the Finnish Defence Forces.
|
|
Allocation concealment
|
high/unclear
|
- Eligible patients were randomly assigned by means of a computer-generated randomization schedule in blocks of 6 to receive 2 inhalations of either zanamivir (Relenza‚Ñ¢, GlaxoSmithKline; 5 mg per inhalation) or matched placebo (lactose powder) twice daily (morning and evening) for 5 d: a total daily dose of 20 mg.
- For viral load measurements, throat and nasopharyngeal swabs were taken at baseline and at 24 h after the first dose, with additional throat swabs being taken at 8 and 48 h. All swabs were taken before study medication was used, kept at 4°C, vortexed and then divided into 2 aliquots for storage at − 70°C.
- This was a randomized, double-blind, placebo-controlled, parallelgroup trial performed at 6 garrisons of the Finnish Defence Forces.
|
|
Blinding of participants and personnel
|
low
|
- This was a randomized, double-blind, placebo-controlled, parallelgroup trial performed at 6 garrisons of the Finnish Defence Forces.
- Eligible patients were randomly assigned by means of a computer-generated randomization schedule in blocks of 6 to receive 2 inhalations of either zanamivir (Relenza‚Ñ¢, GlaxoSmithKline; 5 mg per inhalation) or matched placebo (lactose powder) twice daily (morning and evening) for 5 d: a total daily dose of 20 mg.
- The treatment groups were similar with respect to demographic details.
|
|
Blinding of outcome assessment
|
low
|
- This was a randomized, double-blind, placebo-controlled, parallelgroup trial performed at 6 garrisons of the Finnish Defence Forces.
- The collection and handling of specimens was standardized by laboratory meetings of study doctors and nurses before both influenza seasons.
- A randomized, double-blind, placebo-controlled, parallel-group trial performed at 5 residential units of the Finnish Defence Forces was conducted to assess the antiviral activity, efficacy and safety of inhaled zanamivir for the treatment of naturally acquired influenza.
|
Kohno S, 2010
|
Population
|
- A total of 300 previously healthy adult subjects aged 20 to 64 years with a positive influenza virus rapid antigen test were recruited within 48 h of the onset of influenza symptoms and randomized to three groups: single intravenous infusion of either 300 mg peramivir per kg of body weight, 600 mg peramivir, or matching placebo on study day 1.
- Exclusion criteria included respiratory dysfunction or chronic respiratory disorders requiring pharmacotherapy, convulsions or other neurological symptoms, active clinically important chronic illness or known infection with human immunodeficiency virus, renal impairment requiring hemodialysis, suspected bacterial infection, treatment with steroids or other immunosuppressants, use of anti-influenza virus drugs within the past 7 days; and a history of hypersensitivity , allergy, or serious adverse drug reactions to anti-influenza virus drugs or acetaminophen.
|
|
Intervention
|
- Subjects were randomly assigned to receive a single dose of intravenous peramivir (300 mg or 600 mg) or matching placebo (Shionogi & Co., Ltd., Osaka, Japan).
- A total of 300 previously healthy adult subjects aged 20 to 64 years with a positive influenza virus rapid antigen test were recruited within 48 h of the onset of influenza symptoms and randomized to three groups: single intravenous infusion of either 300 mg peramivir per kg of body weight, 600 mg peramivir, or matching placebo on study day 1.
- The efficacy of peramivir was evaluated by comparing the treatment group (consisting of the 300-mg and 600-mg groups) with the placebo group.
|
|
Outcomes
|
- Other efficacy endpoints included a change (from baseline) in composite symptom scores at 24, 36, 48, and 96 h after the start of treatment; the proportion of afebrile subjects (37°C; axillary); a change in the influenza virus titer from baseline; time to resumption of usual activities; and incidence of influenzarelated complications (otitis media, bronchitis, sinusitis, and pneumonia).
- The primary efficacy endpoint (time to alleviation of symptoms) was defined as the time from the start of treatment to recovery (i.e., when all seven influenza symptom scores had been at " 0 " or " 1 " for at least 21.5 h).
- The presence of the seven influenza symptoms was self-assessed on a 4-point scale (0, absent; 1, mild; 2, moderate; 3, severe) (influenza symptom severity scale [ISS]) and recorded twice daily from day 1 to day 9 and once daily from day 10 to day 14 (16).
|
|
Bias
|
Judgement
|
Support for judgement
|
|
Random sequence generation
|
low
|
- Computer-generated randomization was conducted by a central randomization facility with sole access to the code, using a minimization method to balance current smoking behavior at screening and composite symptom scores at screening among the three groups.
- Each center dispensed the study drug, which was unrecognizable without a drug number, according to the instructions of the randomization center, using assigned randomization numbers.
- A total of 300 previously healthy adult subjects aged 20 to 64 years with a positive influenza virus rapid antigen test were recruited within 48 h of the onset of influenza symptoms and randomized to three groups: single intravenous infusion of either 300 mg peramivir per kg of body weight, 600 mg peramivir, or matching placebo on study day 1.
|
|
Allocation concealment
|
low
|
- Each center dispensed the study drug, which was unrecognizable without a drug number, according to the instructions of the randomization center, using assigned randomization numbers.
- Computer-generated randomization was conducted by a central randomization facility with sole access to the code, using a minimization method to balance current smoking behavior at screening and composite symptom scores at screening among the three groups.
- The study drug, a single intravenous infusion of 30-to 60-min duration, was administered by the appropriate personnel at each center.
|
|
Blinding of participants and personnel
|
low
|
- Virological testing and laboratory tests were performed at BML Corporation (Saitama, Japan) by technicians blinded to treatment assignment.
- A total of 300 previously healthy adult subjects aged 20 to 64 years with a positive influenza virus rapid antigen test were recruited within 48 h of the onset of influenza symptoms and randomized to three groups: single intravenous infusion of either 300 mg peramivir per kg of body weight, 600 mg peramivir, or matching placebo on study day 1.
- This study was a randomized, double-blind, placebo-controlled trial conducted at Nagasaki University Hospital, Nagasaki, and 74 other centers in Japan between December 2007 and April 2008.
|
|
Blinding of outcome assessment
|
low
|
- Computer-generated randomization was conducted by a central randomization facility with sole access to the code, using a minimization method to balance current smoking behavior at screening and composite symptom scores at screening among the three groups.
- Virological testing and laboratory tests were performed at BML Corporation (Saitama, Japan) by technicians blinded to treatment assignment.
- Analysis was performed for observed data on the subset of subjects who were positive for influenza virus at baseline.
|
Watanabe A, 2010
|
Population
|
- The exclusion criteria were as follows: suspicion of infection with a bacterial species or noninfluenza virus within 1 week before enrollment; reported occurrence of any influenza-like symptom within 1 week before the onset of influenza; chronic respiratory disease; renal dysfunction ; history of alcohol or drug abuse; or treatment with amantadine, zanamivir, or oseltamivir within 4 weeks.
- Eligible patients were aged 20 years who presented within 36 h after the onset of influenza symptoms and who had an axillary temperature of 37.5C and a positive test result with use of a rapid influenza diagnostic kit.
- Pregnant women, breast-feeding women, and women who wished to become pregnant during the period of the trial were also excluded.
|
|
Intervention
|
- Laninamivir octanoate was inhaled once on day 1, and oseltamivir (75 mg) was administered orally twice daily for 5 days.
- We conducted a randomized controlled trial to determine whether the efficacy of a single inhalation of laninamivir octanoate was noninferior to that of oseltamivir administered with multiple oral doses in treating adults with seasonal influenza.
- Patients were randomly assigned (1:1:1) to a 40-mg laninamivir octanoate, a 20-mg laninamivir octanoate, or an oseltamivir treatment group.
|
|
Outcomes
|
- The primary end point was the time to illness alleviation.
- The primary end point was the time to illness alleviation, defined as the time from the initiation of trial treatment to the beginning of the first 21.5-h period in which all influenza symptoms were " absent " or " mild. "
|
|
Bias
|
Judgement
|
Support for judgement
|
|
Random sequence generation
|
low
|
- The computer-generated block random allocation sequence was provided by Acronet Corporation and was stratified according to the institution and type of influenza virus on the basis of the results of a rapid diagnostic kit capable of separately detecting influenza A and B.
- The patients, investigators, and trial personnel were blinded to the allocation sequence throughout the trial with use of a double-dummy method.
- Patients were randomly assigned (1:1:1) to a 40-mg laninamivir octanoate, a 20-mg laninamivir octanoate, or an oseltamivir treatment group.
|
|
Allocation concealment
|
low
|
- The patients, investigators, and trial personnel were blinded to the allocation sequence throughout the trial with use of a double-dummy method.
- The computer-generated block random allocation sequence was provided by Acronet Corporation and was stratified according to the institution and type of influenza virus on the basis of the results of a rapid diagnostic kit capable of separately detecting influenza A and B.
- Most patients were infected with influenza A (645 patients with H1N1 and 322 patients with H3N2), and only 3 patients were infected with influenza B. All the H1N1 strains carried the H274Y mutation except for viruses from 2 patients.
|
|
Blinding of participants and personnel
|
low
|
- The patients, investigators, and trial personnel were blinded to the allocation sequence throughout the trial with use of a double-dummy method.
- This multicenter, double-blind, randomized controlled trial was conducted from November 2008 through March 2009 at 117 institutions in Japan, Taiwan, Korea, and Hong Kong.
- Patients whose influenza symptoms had not been alleviated at the time of their withdrawal from the study or at the end of the observation period were censored.
|
|
Blinding of outcome assessment
|
low
|
- Most patients were infected with influenza A (645 patients with H1N1 and 322 patients with H3N2), and only 3 patients were infected with influenza B. All the H1N1 strains carried the H274Y mutation except for viruses from 2 patients.
- The patients, investigators, and trial personnel were blinded to the allocation sequence throughout the trial with use of a double-dummy method.
- For the baseline assessment, the subtype of influenza was determined based on an amplified DNA size by a reverse transcription polymerase chain reaction (RT-PCR) with subtype-specific primers designed from the hemagglutinin sequences of the seasonal H1N1, H3N2, and B viruses.
|
Duval X, 2010
|
Population
|
- Methods Patients
- Exclusion criteria were vaccination against influenza during the 2008–2009 season, recent exacerbations of chronic obstructive pulmonary disease (COPD), asthma or severe chronic disease, previous history of depression, and prior inclusion in this trial.
|
|
Intervention
|
- Adults who visited their general practitioner with symptoms of an influenza-like illness for less than 36 hours and who had a positive influenza A rapid test were randomized to one of three arms: (1) oral oseltamivir 75 mg twice daily plus zanamivir 10 mg by inhalation twice daily, (2) oral oseltamivir 75 mg twice daily plus inhaled placebo, or (3) zanamivir 10 mg by inhalation twice daily plus oral placebo.
- The objective of this study was to compare the short-term virological efficacy of oseltamivir-zanamivir combination versus each monotherapy plus placebo.
- Oseltamivir dosage was 75 mg orally twice daily; zanamivir dosage was 10 mg by oral inhalation using the commercialized GlaxoSmithKline Diskhaler, twice daily.
|
|
Outcomes
|
- Other endpoints were (1) the decrease of log 10 viral load between days 0 and 2 in the patients with confirmed influenza A on day 0 and available samples both at days 0 and 2; (2) the time to resolution of illness; (3) the number of patients with alleviation of symptoms at the end of treatment (day 5); (4) the symptoms score at the end of treatment; (5) the incidence of secondary complications of influenza such as otitis, bronchitis, sinusitis, pneumonia, and the use of antibiotics; (6) the occurrence of adverse events in all participants having received at least one dose.
- Clinical Response Oral temperature was recorded and severity of seven symptoms (nasal stuffiness, sore throat, cough, muscle aches, tiredness or fatigue, headache, and feverishness) was rated by the patient twice daily (morning and evening) up to day 5 and then once daily on a four-point scale (0, none; 1, mild; 2, moderate; 3, severe) [2][3][4]14].
- , we enrolled throughout France adults 18 y old and older who consulted their general practitioner within 36 h of influenza symptoms onset (following the first influenza symptoms reported by the patient), with a temperature greater than or equal to 38uC (reported or observed by the practitioner), one or more respiratory symptoms (cough, sore throat), one or more general symptoms (headache, dizziness, myalgia, sweats and or chills, fatigue), and a positive nasal rapid test for influenza A (
|
|
Bias
|
Judgement
|
Support for judgement
|
|
Random sequence generation
|
low
|
- A computer random number generator was used to select random permuted blocks of size 3.
- Patients were allocated to treatment by a randomization list, with an arm ratio of 1:1:1, balanced by practitioner.
- Adults who visited their general practitioner with symptoms of an influenza-like illness for less than 36 hours and who had a positive influenza A rapid test were randomized to one of three arms: (1) oral oseltamivir 75 mg twice daily plus zanamivir 10 mg by inhalation twice daily, (2) oral oseltamivir 75 mg twice daily plus inhaled placebo, or (3) zanamivir 10 mg by inhalation twice daily plus oral placebo.
|
|
Allocation concealment
|
low
|
- This randomisation code was given to the central hospital pharmacy that prepared blinded treatment units in conformity with good manufacturing practices (GMP).
- Allocation was concealed through the similarity of all the containers and the impossibility for the GP to identify the treatment arm when opening the container.
- Patients, general practitioners assigning the patients, and outcome assessors (practitioners, virologists, patients), were blinded to treatment assignment throughout the study and statisticians until the end of the analysis.
|
|
Blinding of participants and personnel
|
low
|
- In the study, patients, general practitioners, and outcome assessors were all blinded to treatment assignments.
- Patients, general practitioners assigning the patients, and outcome assessors (practitioners, virologists, patients), were blinded to treatment assignment throughout the study and statisticians until the end of the analysis.
- This randomisation code was given to the central hospital pharmacy that prepared blinded treatment units in conformity with good manufacturing practices (GMP).
|
|
Blinding of outcome assessment
|
low
|
- In 2006, in the context of pandemic planning, we designed a double-placebo randomized controlled trial in patients presenting with seasonal influenza-like illness to compare the oseltamivirzanamivir combination to each of the monotherapies plus placebo.
- This randomisation code was given to the central hospital pharmacy that prepared blinded treatment units in conformity with good manufacturing practices (GMP).
- In 2008, they were considered an important strategy to limit the impact of an influenza pandemic both individually, by reducing morbidity and mortality, and collectively, by slowing spread of the virus to allow time for vaccine production, the cornerstone of influenza control [2][3][4]7].
|
Kohno S, 2011
|
Population
|
- Patients aged 20 years or older with influenza A or B virus infection who met the following inclusion criteria were enrolled: availability for treatment within 48 h of onset of influenza symptoms, fever with an axillary temperature of 38.0°C, at least two moderate to severe symptoms among seven symptoms (headache, muscle or joint pain, feverishness or chills, fatigue, cough, sore throat, and nasal stuffiness) due to influenza, and a rapid antigen test (RAT) result positive for influenza.
- Exclusion criteria were impaired respiratory function, a history of congestive cardiac failure, poorly controlled diabetes mellitus, immunosuppressive therapy (immunosuppressants, antitumor agents, etc.) or an immunodeficiency disorder such as AIDS, renal disorder (estimated creatinine clearance, 50 ml/min), ischemic heart disease or serious arrhythmia, a corrected QT interval (QTc) of 480 ms or bradycardia (heart rate, 40 beats per minute [bpm]), clinically significant disorders that required hospitalization, and infection requiring systemic antimicrobial treatment.
- In a multinational, multicenter, double-blind, double-dummy randomized controlled study, patients aged >20 years with influenza A or B virus infection were randomly assigned to receive either a single intravenous infusion of peramivir (300 or 600 mg) or oral administration of oseltamivir (75 mg twice a day [b.i.d.] for 5 days).
|
|
Intervention
|
- In a multinational, multicenter, double-blind, double-dummy randomized controlled study, patients aged >20 years with influenza A or B virus infection were randomly assigned to receive either a single intravenous infusion of peramivir (300 or 600 mg) or oral administration of oseltamivir (75 mg twice a day [b.i.d.] for 5 days).
- Oseltamivir was administered orally at a dose of 75 mg twice daily for 5 days.
- Thus, a single intravenous dose of peramivir may be an alternative to a 5-day oral dose of oseltamivir for patients with seasonal influenza virus infection.
|
|
Outcomes
|
- In addition, the following secondary endpoints were assessed: (i) change from baseline in the composite symptom score, (ii) proportion of patients whose body temperature returned to normal (37.0°C), (iii) time to resumption of usual activities (defined as the first time point when the IIWS score was 10 [able to perform all usual activities fully]), (iv) incidence of influenza-related complications (sinusitis, otitis media, bronchitis, and pneumonia), and (v) time-weighted change from baseline in the virus titer.
- Patients assessed their own influenza symptoms and daily living activities using an Influenza Symptom Severity scale (ISS) (0, none [normal]; 1, mild [of little concern]; 2, moderate [very uncomfortable]; 3, severe [intolerable] ) for seven symptoms (cough, sore throat, headache , nasal stuffiness, feverishness or chills, muscle or joint pain, and fatigue) and a visual analogue scale (Influenza Impact Well-Being Score [IIWS]) ranging from 0 (unable to perform one's usual activities at all) to 10 (able to perform all usual activities fully) (17).
- The onset of influenza symptoms was defined as the time of the first increase of 1°C from the patient's normal body temperature or the occurrence of at least one of the seven symptoms listed above.
|
|
Bias
|
Judgement
|
Support for judgement
|
|
Random sequence generation
|
low
|
- The time-weighted changes in virus titer for the different groups were compared with the van Elteren test, which was stratified by randomization factors.
- Using a minimization method, patients were randomly assigned in a 1:1:1 ratio to receive peramivir at a dose of 300 or 600 mg (Shionogi, Osaka, Japan) or oseltamivir with a balance of the composite symptom score (14 or 15), current smoking behavior (yes or no), country, and influenza virus type revealed by a RAT for the diagnosis of influenza.
- The other covariates were used as minimization factors to ensure balance in randomization.
|
|
Allocation concealment
|
high/unclear
|
- Our study was a multicenter, double-blind, randomized, controlled study with dynamic allocation using the minimization method and was conducted in 146 medical institutions in Japan, South Korea, and Taiwan from November 2008 to April 2009.
- Blinding was maintained by the double-dummy technique using two placebos identical to peramivir and oseltamivir.
- The other covariates were used as minimization factors to ensure balance in randomization.
|
|
Blinding of participants and personnel
|
low
|
- Our study was a multicenter, double-blind, randomized, controlled study with dynamic allocation using the minimization method and was conducted in 146 medical institutions in Japan, South Korea, and Taiwan from November 2008 to April 2009.
- Blinding was maintained by the double-dummy technique using two placebos identical to peramivir and oseltamivir.
- In a multinational, multicenter, double-blind, double-dummy randomized controlled study, patients aged >20 years with influenza A or B virus infection were randomly assigned to receive either a single intravenous infusion of peramivir (300 or 600 mg) or oral administration of oseltamivir (75 mg twice a day [b.i.d.] for 5 days).
|
|
Blinding of outcome assessment
|
high/unclear
|
- The factors of sex, coexisting disease at baseline, and receipt of drugs before randomization were added as covariates prior to unblinding, because the blind review revealed that these factors may have affected the duration of influenza.
- Our study was a multicenter, double-blind, randomized, controlled study with dynamic allocation using the minimization method and was conducted in 146 medical institutions in Japan, South Korea, and Taiwan from November 2008 to April 2009.
- This study did not have a placebo group, and we could not confirm the clinical and virological efficacy of peramivir against the resistant A/H1N1 influenza virus that was widespread in the 2008-2009 season.
|
30184455.pdf
|
Population
|
- The trial enrolled Japanese adults 20 to 64 years of age with acute influenza from December 2015 through March 2016.
- We now report the results of single-dose baloxavir treatment in otherwise healthy persons with acute influenza from phase 2 and 3 randomized, controlled trials.
- The phase 3 trial (CAPSTONE-1) was a doubleblind , placebo-and oseltamivir-controlled, randomized trial that enrolled outpatients 12 to 64 years of age with influenza-like illness in the United States and Japan from December 2016 through March 2017.
|
|
Intervention
|
- Patients 20 to 64 years of age were randomly assigned, in a 2:2:1 ratio, to receive a single oral dose of baloxavir (40 mg for patients weighing <80 kg or 80 mg for those weighing ‚â•80 kg), oseltamivir at a dose of 75 mg twice daily for 5 days, or matching placebos.
- CONCLUSIONS Single -dose baloxavir was without evident safety concerns, was superior to placebo in alleviating influenza symptoms, and was superior to both oseltamivir and placebo in reducing the viral load 1 day after initiation of the trial regimen in patients with uncomplicated influenza.
- The phase 2 trial was a double-blind, placebo-controlled , dose-ranging, randomized trial (randomization ratio, 1:1:1:1) of single doses of baloxavir (10, 20, or 40 mg) or placebo.
|
|
Outcomes
|
- Clinical and Laboratory Monitoring Patients assessed the severity of seven influenzaassociated symptoms (cough, sore throat, headache , nasal congestion, feverishness or chills, muscle or joint pain, and fatigue) on a 4-point scale (with 0 indicating no symptoms, 1 mild symptoms, 2 moderate symptoms, and 3 severe symptoms) twice daily from enrollment (day 1) to day 9 and once daily on days 10 through 14.
- Measured In both trials, the primary efficacy end point was the time to alleviation of symptoms, defined as the time from the start of the trial regimen to the time when all seven influenza-related symptoms (described above) were rated by the patients as absent or mild for at least 21.5 hours.
- The safety end points included the frequencies and severity of adverse events.
|
|
Bias
|
Judgement
|
Support for judgement
|
|
Random sequence generation
|
high/unclear
|
- Patients 20 to 64 years of age were randomly assigned, in a 2:2:1 ratio, to receive a single oral dose of baloxavir (40 mg for patients weighing <80 kg or 80 mg for those weighing ‚â•80 kg), oseltamivir at a dose of 75 mg twice daily for 5 days, or matching placebos.
- The phase 2 trial was a double-blind, placebo-controlled , dose-ranging, randomized trial (randomization ratio, 1:1:1:1) of single doses of baloxavir (10, 20, or 40 mg) or placebo.
- Patients 12 to 19 years of age were randomly assigned, in a 2:1 ratio, to receive either baloxavir or placebo (on day 1 only).
|
|
Allocation concealment
|
high/unclear
|
- The phase 2 trial was a double-blind, placebo-controlled , dose-ranging, randomized trial (randomization ratio, 1:1:1:1) of single doses of baloxavir (10, 20, or 40 mg) or placebo.
- The phase 3 trial (CAPSTONE-1) was a doubleblind , placebo-and oseltamivir-controlled, randomized trial that enrolled outpatients 12 to 64 years of age with influenza-like illness in the United States and Japan from December 2016 through March 2017.
- Data were compiled by the sponsor and analyzed by a statistician employed by the sponsor .
|
|
Blinding of participants and personnel
|
low
|
- The phase 2 trial was a double-blind, placebo-controlled , dose-ranging, randomized trial (randomization ratio, 1:1:1:1) of single doses of baloxavir (10, 20, or 40 mg) or placebo.
- Two serious adverse events were noted in baloxavir recipients (incarcerated inguinal hernia and aseptic meningitis), but neither was considered to be related to the trial regimen by investigators who were unaware of the trialgroup assignments.
- Patients 12 to 19 years of age were randomly assigned, in a 2:1 ratio, to receive either baloxavir or placebo (on day 1 only).
|
|
Blinding of outcome assessment
|
high/unclear
|
- In patients with paired sequenced samples, PA I38T/M amino acid substitutions were detected after initiation of the trial regimen in 9.7% of 370 baloxavir recipients (all the recipients with these substitutions had influenza A(H3N2) infection), typically at day 5 or later, but in none of 95 randomly selected placebo recipients.
- Acetaminophen was allowed, but no other symptomatic therapies, antiviral agents for the treatment of influenza, or antibiotic agents were allowed , except for the treatment of suspected bacterial infections that developed after enrollment .
- ceuticals; Dr. de Jong, serving on an advisory board and receiving travel support and fees for serving on an independent data and safety monitoring board, paid to his institution, from Janssen, serving on an advisory board and receiving travel support, paid to his institution, from MedImmune, serving on an advisory board and receiving travel support from Shionogi, and serving on an independent data and safety monitoring board and receiving travel support, paid to his institution, from GlaxoSmith- Kline and Vertex Pharmaceuticals; Dr. Sekino, receiving grant support from Daiichi Sankyo; Dr. Portsmouth, Ms. Kawaguchi, Dr. Shishido, Mr. Arai, and Dr. Uehara, being employed by Shionogi; Mr. Tsuchiya, being employed by and holding stock in Shionogi; and Dr. Watanabe, receiving consulting fees and lecture fees from Chugai Pharmaceutical, GlaxoSmithKline, Mitsubishi Tanabe Pharma, Janssen Pharmaceuticals, and Sumitomo Dainippon Pharma, grant support, consulting fees, and lecture fees from Daiichi Sankyo, grant support and lecture fees from Shionogi, grant support and consulting fees from Toyama Chemical, and grant support from Fujifilm Pharmaceuticals, Meiji Seika Pharma, and Kyorin Pharmaceutical.
|
References
- Rederick F et al. The New England Journal of Medicine EFFICACY AND SAFETY OF THE NEURAMINIDASE INHIBITOR ZANAMIVIR IN THE TREATMENT OF INFLUENZAVIRUS INFECTIONS Background The sialic acid analogue zanamivir N. Engl. J. Med. 1997. 337(13); 874-80 PMID: 9302301
- Monto AS et al. Efficacy and safety of the neuraminidase inhibitor zanamivirin the treatment of influenza A and B virus infections. J. Infect. Dis. 1999. 180(2); 254-61 PMID: 10395837
- Treanor JJ et al. Efficacy and safety of the oral neuraminidase inhibitor oseltamivir in treating acute influenza: a randomized controlled trial. US Oral Neuraminidase Study Group. JAMA 2000. 283(8); 1016-24 PMID: 10697061
- Mäkelä MJ et al. Clinical efficacy and safety of the orally inhaled neuraminidase inhibitor zanamivir in the treatment of influenza: a randomized, double-blind, placebo-controlled European study. J. Infect. 2000. 40(1); 42-8 PMID: 10762110
- Boivin G et al. Rapid antiviral effect of inhaled zanamivir in the treatment of naturally occurring influenza in otherwise healthy adults. J. Infect. Dis. 2000. 181(4); 1471-4 PMID: 10762579
- Unable to extract citation information for file 10866439.pdf PMID: not found
- Unable to extract citation information for file 12667386.pdf PMID: not found
- Puhakka T et al. Scandinavian Journal of Infectious Diseases Zanamivir: a Significant Reduction in Viral Load During Treatment in Military Conscripts with Influenza Zanamivir: a Significant Reduction in Viral Load During Treatment in Military Conscripts with Influenza Scand. J. Infect. Dis. 2003. 1(1); 52-58 PMID: 12685885
- Kohno S et al. Efficacy and safety of intravenous peramivir for treatment of seasonal influenza virus infection. Antimicrob. Agents Chemother. 2010. 54(11); 4568-74 PMID: 20713668
- Watanabe A et al. Long-acting neuraminidase inhibitor laninamivir octanoate versus oseltamivir for treatment of influenza: A double-blind, randomized, noninferiority clinical trial. Clin. Infect. Dis. 2010. 51(10); 1167-75 PMID: 20936975
- Duval X et al. Efficacy of oseltamivir-zanamivir combination compared to each monotherapy for seasonal influenza: a randomized placebo-controlled trial. PLoS Med. 2010. 7(11); e1000362 PMID: 21072246
- Kohno S et al. Phase III randomized, double-blind study comparing single-dose intravenous peramivir with oral oseltamivir in patients with seasonal influenza virus infection. Antimicrob. Agents Chemother. 2011. 55(11); 5267-76 PMID: 21825298
- Unable to extract citation information for file 30184455.pdf PMID: not found
個々の研究の結果をまとめてみた。
小さくて読めないので適宜拡大してご覧ください。
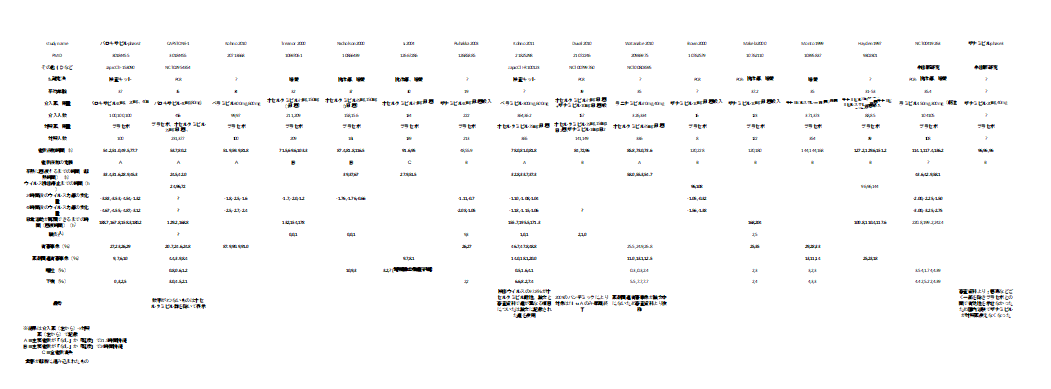
ザナミビルが症状緩和までの時間で有効だったものが採用されていない印象。
(症状緩和までの時間の定義の違いかと考えて、全研究の定義を確認したが除外された理由はわからなかった)
オセルタミビル耐性ウイルスに対するオセルタミビルとの非劣性試験だったのはペラミビルとラニナミビル。そもそも対プラセボで有効性が示されなかったザナミビルが印象的。
ネットワーク図も簡易表記だったので、アウトカムごとに書き直してみた。(n数がわからなかったので丸のサイズはすべて同一です。線の太さのみ研究数を反映しています。研究数はおおよそ1~3です)
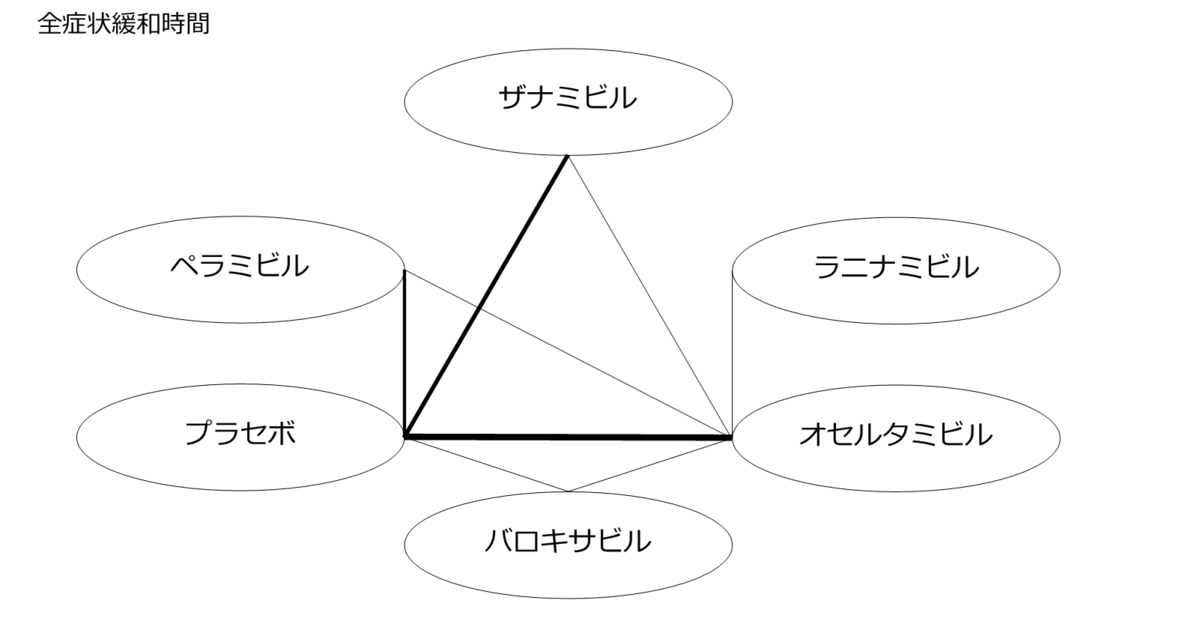
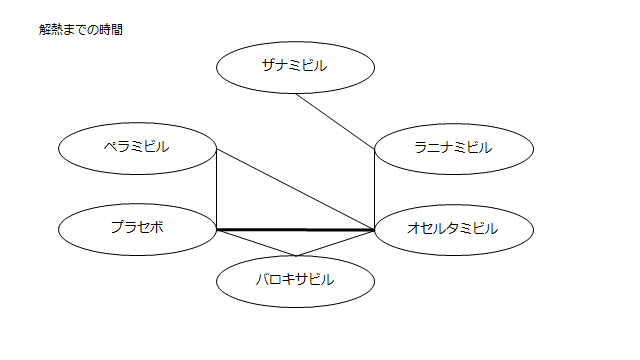
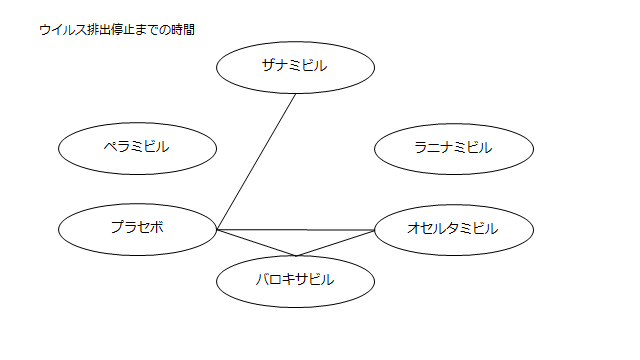
<おまけ>
読んだ瞬間「あれ?IGLOOがない?これじゃラニナミビルの効果を過剰に見積もってしまう」と思い詳しく読み始めたわけですが、なぜラニナミビルにこれほどまで忖度しているのかわからなかった。
COIの項目を読んでみると、「 NH reports personal fees and other from Shionogi & Co., Ltd.,outside the submitted work」とあり、塩野義以外のCOIは書いていない。
以前同医師によるイナビルの講演を聞いたような記憶があったので、COIを調べてみた。
マネーデータベース『製薬会社と医師』~あなたの医者をみつけよう
で、「廣津伸夫」と検索すると
で名前をクリックすると
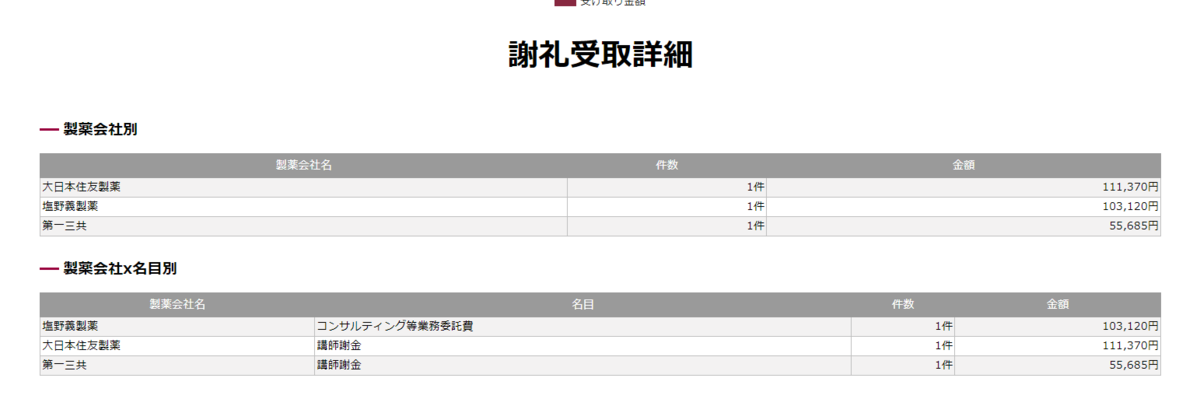
ここから感想
「ちょっと、論文で示されていない第一三共とCOIあるやん!
やたらラニナミビル(イナビル)に忖度したみたいな研究選定されてるから邪推したくなるやん!」
















